Thursday 10th May 2018
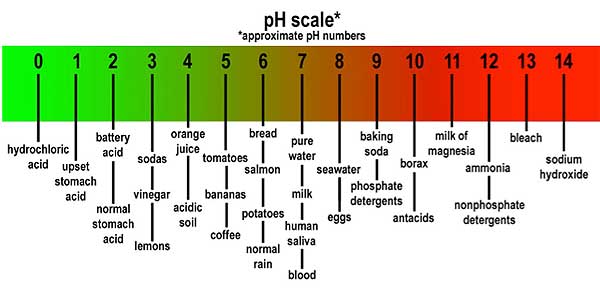 The pH scale determinate the acidity or alkalinity of a solution. The scale range from zero to 14. The zero end of scale is where solutions are very acidic. Moving up around 2 on the scale is the rating for lemon juice. Around 3 is vinegar, beer and cola. Pure water has a pH of 7, which is natural. As you move up the scale from 7 , solutions become more alkaline (some chemicals in this range are commonly referred to as bases).
The pH scale determinate the acidity or alkalinity of a solution. The scale range from zero to 14. The zero end of scale is where solutions are very acidic. Moving up around 2 on the scale is the rating for lemon juice. Around 3 is vinegar, beer and cola. Pure water has a pH of 7, which is natural. As you move up the scale from 7 , solutions become more alkaline (some chemicals in this range are commonly referred to as bases).What are the Variations of pH Between different Cleaners?
Household
Bleach has a pH of 12. Oven cleaners fall between 13 and 14 making these very
strong Alkali Cleaners, on the opposite end of the Scale we have Toilet Cleaners with a pH of 2 and Descalers with a pH of 1 and 2. High pH
Detergents and Cleaners tend to be
used for removing Wax (Floor Polishs) or
items badly soiled with grease. Strong acids (low pH) tend to be used to Descale
and clean items such as toilet bowls or for Deep Cleaning (Etching).
Cleaners, on the opposite end of the Scale we have Toilet Cleaners with a pH of 2 and Descalers with a pH of 1 and 2. High pH
Detergents and Cleaners tend to be
used for removing Wax (Floor Polishs) or
items badly soiled with grease. Strong acids (low pH) tend to be used to Descale
and clean items such as toilet bowls or for Deep Cleaning (Etching).
Cleaning products at either end of the pH scale are often very corrosive and require professional use. This type of power is often necessary to obtain good cleaning results. Products such as washing up liquid and cleaners mainly have a neutral pH value.
What is BS EN 1276 testing?
BS EN 1276 is a European Standard which specifies a test method and the minimum requirements for bactericidal activity of chemical disinfectant and antiseptic products that form a homogeneous, physically stable preparation when diluted with hard water or - in the case of ready-to-use products - with water. BS EN 1276 applies to products that are used in food, industrial, domestic and institutional areas excluding areas and situations where disinfection is medically indicated and excluding products used on living tissues except those for hand hygiene in the above considered areas.



